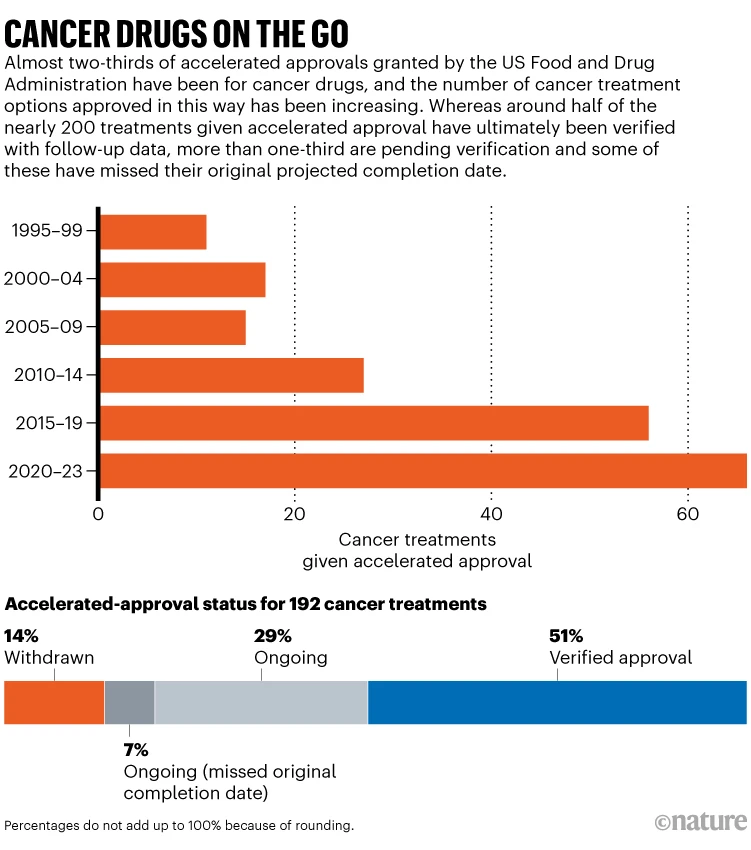
In August 2021, Amol Akhade, an oncologist at Nair Medical Hospital in Mumbai, India, received an e-mail from the Swiss drug manufacturer Roche recommending the use of a drug named atezolizumab to treat a specific kind of breast cancer. Akhade was surprised. That month, Roche had withdrawn the drug for this purpose in the United States (although it is still approved to treat other kinds of cancer).
For the type of breast cancer in question — known as triple-negative because it lacks three key protein markers — atezolizumab was made available in 2019 through the US Food and Drug Administration’s […]
Click here to visit source. www.nature.com
See also Alleged Russia agent pleads not guilty to smuggling US tech, arms
Powered by Inline Related Posts